hermes crystal structure fred dyda1 | Structural Basis of hAT Transposon End Recognition by hermes crystal structure fred dyda1 APC is an RNA-binding protein whose mRNA interactome is highly enriched for . 1. S – IV (subject – intransitive verb) Anna smiled (softly). S IV adv. Tim left (early). S IV adv. Give your own examples. Ms. Perkins is talking (too loudly) S IV. adv. S – LV – C (Subject – Linking Verb – Complement. Helen Keller is my favorite writer. S LV C. The cake was bitter. S LV C. They have been very good friends. S LV C.
0 · Fred Dyda
1 · Structural insights into the mechanism of double strand break
2 · Structural insights into the mechanism of double strand break
3 · Structural basis of hAT transposon end recognition by Hermes, an
4 · Structural Basis of hAT Transposon End Recognition by Hermes,
5 · Structural Basis of hAT Transposon End Recognition by
6 · Structural Basis for Transposon End Recognition by Hermes, an
7 · Publications
8 · Molecular architecture of a eukaryotic DNA transposase
9 · Fred Dyda
Find out more with MyAnimeList, the world's most active online anime and manga community and database. Goblins are known for their ferocity, cunning, and rapid reproduction, but their reputation as the lowliest of monsters causes their threat to be overlooked. Raiding rural civilizations to kidnap females of other species for breeding, .
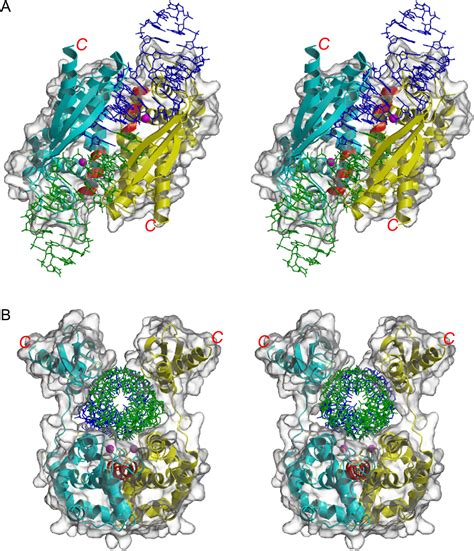
The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active .
Hermes is a member of the hAT transposon su-perfamily that has active .Antigen receptor assembly in lymphocytes occurs by the random rearrangement of .APC is an RNA-binding protein whose mRNA interactome is highly enriched for .
The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active in vitro . The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. While isolated dimers are active in . Here, we describe several co-crystal structures of the Hermes transposase bound to DNA that mimics the reaction step immediately prior to hairpin formation. Our results reveal .The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active.
Biography Pages. Publications. A selection of recent and significant publications can be viewed below. Select Publications. Structural basis of hAT transposon end recognition by . How flanking hairpins are formed during DNA transposition has remained elusive. Here, we describe several co-crystal structures of the Hermes transposase bound to DNA that . The structure of Hermes 79–612 was solved using X-ray crystallography with multiwavelength anomalous dispersion on selenomethionyl-substituted protein (see Methods .
Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferasesA eukaryotic transposase needs to assemble as an octamer to function in vivo. The abundance of subunits provides multiple DNA binding domains that seem to confer greater affinity and . The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active in vitro for all the chemical steps of transposition, only octamers are active in vivo.The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active in vitro for all the chemical steps of transposi-tion, only octamers are active in vivo.
The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. While isolated dimers are active in vitro for all the chemical steps of transposition, only octamers are active in vivo.
Here, we describe several co-crystal structures of the Hermes transposase bound to DNA that mimics the reaction step immediately prior to hairpin formation. Our results reveal a large DNA conformational change between the initial cleavage step and subsequent hairpin formation that changes which strand is acted upon by a single active site.The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active. Biography Pages. Publications. A selection of recent and significant publications can be viewed below. Select Publications. Structural basis of hAT transposon end recognition by Hermes, an octameric DNA transposase from Musca domestica.
Fred Dyda
How flanking hairpins are formed during DNA transposition has remained elusive. Here, we describe several co-crystal structures of the Hermes transposase bound to DNA that mimics the reaction step immediately prior to hairpin formation. The structure of Hermes 79–612 was solved using X-ray crystallography with multiwavelength anomalous dispersion on selenomethionyl-substituted protein (see Methods and Supplementary Fig..
Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases
A eukaryotic transposase needs to assemble as an octamer to function in vivo. The abundance of subunits provides multiple DNA binding domains that seem to confer greater affinity and specificity for transposon mobilization. The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active in vitro for all the chemical steps of transposition, only octamers are active in vivo.The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active in vitro for all the chemical steps of transposi-tion, only octamers are active in vivo.
The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. While isolated dimers are active in vitro for all the chemical steps of transposition, only octamers are active in vivo. Here, we describe several co-crystal structures of the Hermes transposase bound to DNA that mimics the reaction step immediately prior to hairpin formation. Our results reveal a large DNA conformational change between the initial cleavage step and subsequent hairpin formation that changes which strand is acted upon by a single active site.The crystal structure of the Hermes transposase-DNA complex reveals that Hermes forms an octameric ring organized as a tetramer of dimers. Although isolated dimers are active.
Biography Pages. Publications. A selection of recent and significant publications can be viewed below. Select Publications. Structural basis of hAT transposon end recognition by Hermes, an octameric DNA transposase from Musca domestica. How flanking hairpins are formed during DNA transposition has remained elusive. Here, we describe several co-crystal structures of the Hermes transposase bound to DNA that mimics the reaction step immediately prior to hairpin formation. The structure of Hermes 79–612 was solved using X-ray crystallography with multiwavelength anomalous dispersion on selenomethionyl-substituted protein (see Methods and Supplementary Fig..Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases
Structural insights into the mechanism of double strand break
gucci bag sweden

gucci bag style2237
Structural insights into the mechanism of double strand break
Don’t forget to catch us live tomorrow at 2pm on FB! It’s your chance to score this stunning LV Mini Soft Trunk Don’t miss out - 9% OFF STOREWIDE #Glampot
hermes crystal structure fred dyda1|Structural Basis of hAT Transposon End Recognition by